Courier Instructions
Overview
This guide is offered to our contracted courier companies and their drivers to help them safely and legally package and ship medical specimens to Mayo Clinic Laboratories for testing.
Although this document makes many references to regulatory requirements, it is not intended to serve as a comprehensive training program for Department of Transportation (DOT) or International Air Transport Association (IATA) training requirements. We highly recommend that anyone involved in transporting medical specimens become familiar with the many governmental and airline regulations pertinent to these types of shipments. The US DOT Website contains technical information about compliance with the Dangerous Goods regulations. These regulations apply to both ground and air transport. In addition, the IATA Website provides information on airline requirements.
Various federal and international agencies have published rules and regulations that are available on the Internet regarding the transportation of medical specimens. These include:
| ICAO | International Civil Aviation Administration |
|---|---|
| IATA | International Air Transport Association |
| DOT | Department of Transportation |
| CDC | Centers for Disease Control |
| TSA | Transportation Safety Administration |
| FDA | Food and Drug Administration |
| OSHA | Occupational Safety and Health Administration |
| FAA | Federal Aviation Administration |
For more information, call Mayo Clinic Laboratories at 800-533-1710 and ask to speak with a Transportation Specialist.
As regulations, methods, and packaging materials change over time, we urge the reader to obtain the most current information available when shipping medical specimens. Refer to www.mayocliniclabs.com.
Objectives
This guide has been developed to meet the following objectives:
- Provide instructions for packaging specimens
- Provide information about supplies
- Use as a tool for training additional staff members
Specimen Integrity
Specimens must be packed and shipped properly for accurate testing, which helps ensure that patients receive optimal treatment. A specimen may not be viable for testing if it becomes too cold or too hot. It may be necessary to collect another specimen from the patient, which may delay treatment. People’s lives may depend on our work.
Mayo Clinic Laboratories’ goal is to ensure that all medical specimens arrive at the testing facility:
- At the correct temperature for testing
- Intact in the container, without breakage or leakage
- In the shortest possible time
- In compliance with all applicable regulations
This guide has been prepared to help couriers understand their role in accomplishing these goals. By following the guidelines for proper specimen preparation, packing, shipping, and documentation, couriers can comply with regulations and safely transport Mayo Clinic Laboratories specimens.
Courier Support
Mayo Clinic Laboratories Transportation Specialists are available Monday through Friday, 6:00 a.m. to 4:30 p.m. CDT.
For emergency support 24 hours per day, 365 days per year, contact Customer Service.
Packaging Specimens

For convenience, biohazard color-coded, temperature-specific bags are provided to client laboratories by Mayo Clinic Laboratories:
Medical samples being transported to testing laboratories must be maintained at an appropriate temperature: ambient, refrigerate, or frozen.
- Refrigerate Specimen Bag – pink (T229)
- Ambient Specimen Bag – white (T027)
- Frozen Specimen Bag – yellow (T121)
Specimens must be separated by temperature, and each of these temperature bags must be packaged in a specific manner. Mayo Clinic Laboratories provides a raspberry-colored shipping box that holds two Styrofoam (Styro) containers inside. Styro transportation coolers provided by Mayo Clinic Laboratories have corresponding color-coded labels and contain appropriate labeling for dry ice and biohazards. One of the Styro containers holds the cool packs needed to keep refrigerate/ambient specimens cool for up to 48 hours.
All specimens must be shipped in a leak-proof container, regardless of transport temperature. The color-coded bags also contain material that can absorb the full liquid content of the specimens placed inside.
The following pages provide couriers with detailed instructions for packaging specimens for shipping. This includes preparing the Styros, packing specimens at the appropriate temperature (refrigerated, ambient, and frozen), completing shipping documents, and shipping specimens. In addition, the guide includes information about shipping infectious substances, safety in the laboratory, and cleaning up leaks and spills.
Completing the Mayo Clinic Laboratories Specimen Control Document
The following information needs to be filled out completely on the Specimen Control Document that we provide for you:
| Client Name | Account Number | Number of Frozen Bags | Number of Refrigerate Bags | Number of Ambient Bags | Other Material Number and Description | Time of Pick Up | Client Initial |
- On the top section of the control document, on the specified line, print:
- Complete name of the courier company
- Date of the run and day of the week
- Name of the person making the run
- City and state
- Fill in the client name: the names of the hospitals, clinics, or laboratories from which specimens were picked up.
- Fill in the account number: Each client is given an account number by Mayo Clinic Laboratories. If you do not know the account number, our Transportation Department can provide you with that information.
- Mark down the number of bags you picked up for each temperature as well as any other material you may get, such as X-rays or envelopes.
- Log the time you picked up the specimens and have a lab employee initial the Specimen Control Document, stating you have picked up everything.
On-Call Clients
Check with your dispatch department for any on-call clients who may have called in requesting a pickup. On-call clients are those clients not on your regular daily route.
- Determine the most efficient way to include the on-call clients into your scheduled route.
- Add the on-call client information to the control document as you would for a regular pickup.
The Specimen Control Document is a two-part form. Place the original in the Refrigerate/Ambient Styro and keep the copy for your records for future reference.
Preparing the Styros
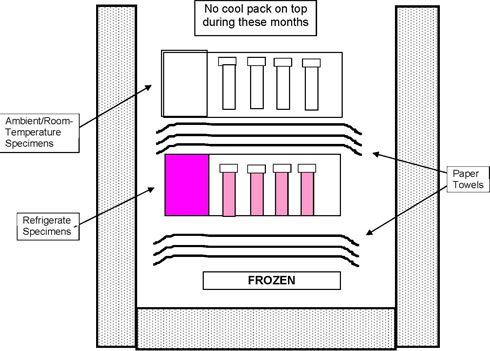
Prepare to pack the refrigerated/ambient specimens in the same Styro. The lid of the Styro has a pink label to indicate Refrigerate Specimens and a white label to indicate Ambient Specimens. Specimens should be in temperature color-coded bags that should match the color of the label on the Styro lid. Put on gloves prior to placing the specimen bags into the Styros.
Packing Refrigerated Specimens
- Place a cold cool pack in the bottom of a Styro.
- Place three to four paper towels (for insulation) over the cool pack.
- Locate the refrigerated specimens and count the bags. Make sure all the bags are pink.
- Record the number of refrigerated bags on the Specimen Control Document.
- Open the lid of the refrigerated/ambient Styro. Remove any ambient specimens from previous pickups.
- Insert the pink refrigerated specimen bags in the Styro. Place two paper towels over the refrigerated specimens, separating them from the ambient specimens. Replace the ambient specimens.
- Immediately replace the Styro lid.
Packing Ambient Specimens
Use the same refrigerated Styro, however:
- Locate the ambient specimens and count the bags. Make sure all the bags have white labels.
- Record the number of ambient bags on the Specimen Control Document.
- Open the lid of the refrigerated/ambient Styro and place two paper towels over the top of the pink refrigerated bags.
- Insert the white ambient specimen bags. Ambient specimens go on top of the refrigerated specimens, separated by paper towels.
- Fill the remaining space inside the Styro with packing material, such as paper towels.
- From November through March, also place a nonrefrigerated cool pack at the top of the Styro.
- Immediately replace the Styro lid.
Refrigerate and Ambient Shipping Container - November through March
Diagram 1a

Refrigerate and Ambient Shipping Container - April through October
Diagram 1b
The frozen cool pack helps keep specimens from becoming too warm.
Reminder: Do not use dry ice to freeze the cool pack. If dry ice is your only option, then remove the cool pack from the dry ice three hours before use.
Packing Frozen Specimens
Note: Pellet dry ice is preferred. Block dry ice must be reduced to small pieces (1 to 2 inches) to minimize the chance of damage to bags and vials.
- Prepare the frozen Styro. The lid of the Styro will have a yellow label to indicate frozen specimens.
- Locate the frozen specimens and count the bags. Make sure all the bags have yellow labels.
- Record the number of frozen bags on the Specimen Control Document.
- Open the lid of the frozen Styro and place a 2-inch layer of dry ice in the bottom of the frozen Styro.
Important: To avoid burns, always wear cloth gloves and safety glasses when handling dry ice. - Insert the yellow specimen bags on top of the dry ice. Add bags only up to the line marked on the yellow sticker inside of the Styro. Cover them with more dry ice. Do not overfill, leaving enough room at the top to put the lid on securely.
- Immediately place the yellow-labeled Frozen cover onto the Styro.
Under normal conditions, 2.2 kg (5 lb), of dry ice will keep the specimens frozen until they arrive at the laboratory.
Frozen Shipping Container
Diagram 1c

Reminder: Do not fill the frozen styrofoam shipping container too full of specimen bags. Leave room for dry ice on top of the topmost specimen shipping bag and make sure the styrofoam lid fits tightly. On passenger aircraft, such as Delta or American, put 2.2 kg (5 lb) of dry ice in the Styro. FedEx or AirNet allow more, because there is a higher maximum weight for dry ice on a cargo aircraft.
Packing Stool Specimens
Mayo Clinic Laboratories provides clients with special containers for stool specimens. We prefer that stool specimens be packed in these containers. Any substitute containers must have a screw cap and be leakproof. A special 10-lb Styro in its own box is used for shipping one or two stool containers. Large-volume stool containers must not be mixed with other specimens. They must be shipped in a separate Styro. Special stool containers may be located in the client’s freezer or refrigerator.
The procedure for packing stool containers is as follows:
- After packing all other specimens, couriers should discard latex gloves, wash their hands, and prepare a 10-lb Styro out in the transport vehicle (if there are more than two stool containers to pick up, use both Styros in a full-sized double 10-lb box set).
- If the stool containers are in the freezer, put a 2-inch layer of dry ice in the bottom of the Styro. If the stool containers are in the refrigerator, put a cold cool pack in the Styro with two paper towels on top.
- Bring the stool container box into the lab. Put on a fresh pair of latex gloves before entering the lab.
- Prepare a Specimen Control Document for this Styro. Fill out the top portion of the document (company name, the name and city shipping from, and the date). Fill in the client’s name and account number.
- Write the number of containers packed in this Styro under the appropriate column: frozen or refrigerated.
- Pack the stool containers in the 10-lb Styro.
Remember: No other type of specimen can be packed in the same Styro with stool containers.
Packing Muscle Biopsy Specimens
Mayo Clinic Laboratories provides clients with special containers for muscle biopsy specimens. A special 10-lb Styro in its own box is used for shipping these biopsies in a small container, completely surrounded by dry ice. They must be shipped as packaged by the client and must not be opened unless specifically directed by the client or by Mayo Clinic Laboratories (for example, to add dry ice in the case of a delayed shipment).
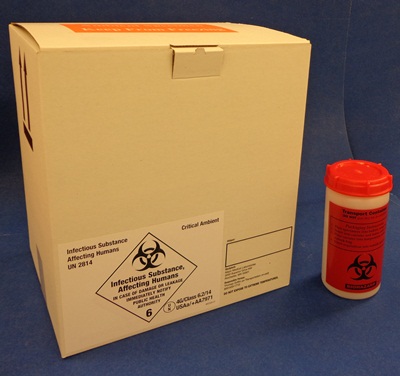
Packing Critical Ambient Specimens
Mayo Clinic Laboratories provides clients with special containers for critical ambient specimens, such as bone marrow and some whole blood specimens that would be ruined if allowed to get too cold.
A 10-lb Styro inside a white fibreboard shipping box (T668) is used for shipping these critical ambients, which are completely surrounded by six room-temperature cool packs. They must be shipped as packaged by the client, and should not be opened by the courier unless specifically directed to do so by the client or by Mayo Clinic Laboratories. If the client has critical ambient specimens but does not have Mayo Clinic Laboratories’ Critical Ambient white box, the courier should package those bags surrounded by six room-temp cool packs in a separate Styro and box.
Packing Other Materials
Sometimes materials that do not fit into Mayo Clinic Laboratories specimen bags or specimen containers must also be packed and transported. Examples include a box of glass slides or X-ray films in a large envelope. Following are instructions for preparing these materials:
- Write the number of items and a description of each item on the Specimen Control Document under Other Material.
- Place the materials in the appropriate Styro, usually ambient/refrigerated. It may be necessary to use an extra box.
- Place envelopes inside of the Styro if they fit. Do not place envelopes alongside the Styros in the box.
- Ship X-ray films or envelopes that do not fit in the Styro via FedEx in a separate X-ray envelope. Mayo Clinic Laboratories provides special X-ray envelopes to couriers and clients who request them. The supply number is T531.
Labeling Culture Specimens
You will see a blue “C” sticker on the bag and batch sheet of any culture specimen. In this case, place a blue “C” sticker on the Styro containing the culture and also on the outside of your berry box. Be sure not to obscure any other labels on the box.

Properly placed C stickers
General Instructions for Packing Specimens
- Never take the lids off both Styros at the same time. When both lids are removed, it is easy to mistakenly place the lids on the wrong Styros.
- Never overfill a Styro or compress the specimens. If the lid will not fit securely on a Styro, use another box.
- Complete a specimen control document for each box, logging only the specimens in that box.
- Count the number of bags and enter that number on the Specimen Control Document.
- If specimens are not in Mayo Clinic Laboratories bags or containers, ask a lab staff person to repackage them. Couriers should pack extra bags in case clients have used up their supplies. (Clients may order supplies by following their normal reorder process.)
- If the temperature of a specimen does not match its label (for example, if a specimen with a pink refrigerated label is in the freezer), ask a laboratory staff person how it should be transported. If it is in the wrong bag or container, ask the person to repackage it.
- Do not leave a box containing specimens in an unlocked car. Always enter a facility carrying the box.
- Do not place any documents, packages, envelopes, and so forth, outside of the Styros in the box.
Pack Extra Cool Packs
Put at least three extra cold cool packs and a dozen paper towels in the refrigerate/ambient Styro of another berry box. It may be necessary to ship more than one refrigerated Styro.
Important: Refrigerated specimens cannot be shipped without a cold cool pack, so it is very important to have extras.
For additional assistance or questions regarding specimen packaging, supplies, or shipping, contact Mayo Clinic Laboratories at 800-533-1710 or visit www.mayocliniclabs.com.
Safety in the Laboratory
Mayo Clinic Laboratories packaging materials are designed to protect everyone who handles them. However, couriers should always take special precautions in a laboratory:
- Wear latex gloves in the laboratory. Cover any cuts or scrapes with a bandage.
- Wash hands before leaving each laboratory.
- Open-toed shoes are not acceptable in the laboratory areas.
- Do not put the box down in a spill (wet) area.
- Do not touch any specimen bag or container that appears soiled. Ask a laboratory staff person to place the specimens in another bag for safe transportation.
- In the event of a cut or puncture to the skin in the lab, tell a laboratory staff person immediately.
Packing the Courier Vehicle
Pack the courier vehicle with the following items (more about some of these items later):
- Extra dry ice with cloth gloves and safety glasses
- Box of latex gloves (provided by Mayo Clinic Laboratories)
- Paper towels
- Cleanup kit
- Packaging tape
- Class 6 Infectious Substance shipping container (T570)
- Mayo Clinic Laboratories stool containers in a 10-lb Styro
- Double 10-lb box sets with cool pack and dry ice
- Specimen Control Document (be sure to pack extras)
- Airbills
- Mayo Clinic Laboratories Shipment Alert Procedure instruction card for calling Mayo Clinic Laboratories to report the shipment
- Mayo Clinic Laboratories phone number 800-533-1710. Ask for the Transportation Department. Phone coverage is available 24 hours a day, 7 days a week.
Important: Always pack the boxes with the cool packs and dry ice just before leaving. Cool packs can freeze if left in a very cold car and dry ice can evaporate if left in a very warm car.
Shipping Documents
Fill out an airbill for every shipment.

Leaving the Laboratory
After all the specimens are securely packed and logged:
- The courier should have the client initial the Specimen Control Document. Note the time of pickup.
Important: The Specimen Control Document is the courier's record of what was picked up. Having the client initial this record is very important. - The client may have a log to fill out as well. If so, do this in addition to filling out the Specimen Control Document.
- Discard gloves in a biohazard trash container. (Ask the client where this is.)
- Wash your hands.
Shipping Instructions
Preparing the Shipping Box
- Cover the specimens in the frozen Styros with dry ice.
- Wear safety glasses and cloth gloves when handling dry ice.
- If no frozen specimens are being shipped, carefully transfer the dry ice from the frozen Styro to the dry ice container and ship that Styro empty.
- The courier may wish to dispose of the extra dry ice after topping off the frozen Styros. The last client on the run may have a place to do this.
Important: Never leave dry ice where people might burn themselves on it. Do not dump it in a parking lot, a drinking fountain, or a restroom sink.
- Put the Specimen Control Document in the Refrigerate/Ambient Styro.
- First, total the columns on each Specimen Control Document. Remove and keep the yellow copy for your records. This will be very important if there should be any questions about the shipment.
- Put the Specimen Control Document inside of the Refrigerate Styro before closing the box.
- Close the shipping box and seal it with packaging tape.
- Do not fold the handles down and tape the box until finished with all pickups.
- Do not seal all the edges of the box. Dry ice emits a gas, which must be allowed to escape. Do not tape the Styro lids.
- On the outside of the box, write the weight (in kilograms) of dry ice being
shipped.
- Usually shipments include some frozen specimens. The Mayo Clinic Laboratories’ shipping box has the words Dry Ice next to the Class 9 hazard label and includes a place to write in the weight of the dry ice (in kilograms) on the box.
Important: If the shipment does not include dry ice, the dry ice label must be completely covered up either with tape, paper, or a marker. - If a shipment containing dry ice is being transported on a passenger airline, write "2.2 kg" on the appropriate line on the label. Make sure that there is no more than 2.2 kilograms (5 lb) of dry ice in the frozen Styro inside the box.
- Usually shipments include some frozen specimens. The Mayo Clinic Laboratories’ shipping box has the words Dry Ice next to the Class 9 hazard label and includes a place to write in the weight of the dry ice (in kilograms) on the box.
This is a Mayo Clinic Laboratories box properly labeled for a Biological Substance, Category B shipment.
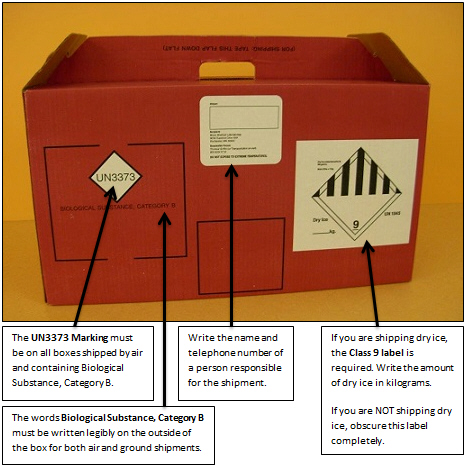
Always ship both Styros in the Mayo Clinic Laboratories box set, even if one Styro is empty. Never take one or both Styros out and put specimens directly in the box. Wear latex gloves any time you reach into a Styro that contains specimens.
Shipping by Air
Completing the Airbill
Mayo Clinic Laboratories will provide couriers with preprinted airbills for shipments or Category B substances.
Mayo Clinic Laboratories’ policy is that Category A Infectious Substances must be shipped by FedEx or AirNet. That procedure will be covered in the section on Category A Infectious Substances. Biological Substance, Category B specimens may generally be shipped by most major passenger airlines as well as by FedEx and AirNet.
Dropping Off and Reporting Your Shipment
The end of the courier’s route is the airline's drop-off location. Take the shipping boxes inside and hand them over to the airline's receiving person.
After delivering the shipment to the airline, call Mayo Clinic Laboratories to report the shipment. If no phone is handy at the airline's office, couriers can do this later from another phone. Mayo Clinic Laboratories’ "AUDIX call-in system" makes it very easy to report the shipment. These instructions have been printed on a handy wallet card as well.
- Call 877-508-9797.
- After the recording, clearly and slowly state your name, company, city, state, flight number, airbill number, name of the airline, and the number of boxes sent on each airbill.
- Hang up.
Important: It is important to make this call within a few hours. Mayo Clinic Laboratories will need this information urgently if the shipment doesn't arrive on time.
Packaging Category A Infectious Substances
The following information describes Mayo Clinic Laboratories' methods of packaging and shipping Category A Infectious Substances. We also provide detailed Dangerous Goods Training.
The packaging certified to transport infectious specimens legally and safely is a combination package consisting of inner and outer containers that have passed all tests required by IATA and DOT. Mayo Clinic Laboratories provides a certified shipping container (T570).
This combination package is certified to transport Category A Infectious Substances only if it is used properly. The box and the inner container by themselves are not considered certified.
Certified packaging for Category A specimens: Infectious Shipper and Infectious Container.
Write the courier's name and address in the space provided on the shipper (box).
Client Preparation
It is not up to the courier driver to decide what is Category A Infectious Substance or Biological Substance, Category B. The physician or send-out staff at the hospital lab will perform that function. Couriers will know that they are handling and transporting an infectious specimen if the client has a UN-certified box for them to ship.
The client places the specimen in a primary receptacle (such as a tube or a vial) and puts the primary receptacle into a certified container. The container is placed in one of Mayo Clinic Laboratories’ color-coded bags. The client will place the bagged container in the white certified box.
Packing Category A Infectious Substances
Do not package infectious Category A Infectious Substance specimens with Biological Substance, Category B specimens. Category A Infectious Substance specimens must be shipped in certified packaging. Because many passenger airlines have restrictions or exclusions on shipping Dangerous Goods, Mayo Clinic Laboratories recommends that any infectious shipments sent to our laboratories be made via FedEx.
If the courier uses a passenger airliner to ship the Category B shipments, the infectious specimens must be shipped to Mayo Clinic Laboratories via FedEx.
Follow this procedure:
- When making a pickup at a hospital, place the certified containers with infectious specimens inside a certified T570 box.
- Remember that shipping the infectious box via air will involve filling out a Shipper’s Declaration as well as a paper FedEx airbill.
- The Category B Biological Substance specimens may be shipped as usual via the courier’s scheduled carrier in another box.
Labeling Category A Infectious Substance Shipments
The courier must write the courier’s name and address on the space provided on the box.
Documenting Category A Infectious Substances
Fill out the required sections of the Shipper's Declaration, including:
- The airbill number
- The airport of departure
- The printed name and title of the person making the shipment, city, state, date, and the signature of the person making the shipment
Shipper's Declaration for Dangerous Goods
Leaks and Spills
To be safe, treat every spill as if it were infectious. If any specimen container in the laboratory appears to be leaking, do not touch it. Bring it to the attention of lab personnel for repackaging. Do this even if the leaking container is inside another bag.
If something spills in the lab, immediately bring it to the attention of a laboratory staff person. Apologize, but let the laboratory staff clean it up.
If a leak or spill occurs away from a laboratory, the courier will have to clean it up, using either a commercial cleanup kit or bleach as follows:
- Make sure no one touches or walks through the spill.
- Always wear latex gloves when dealing with a spill.
- If a spill is large, blot up as much as possible with a paper towel.
- Any materials used to clean up a spill, including paper towels and gloves, should be sent to Mayo Clinic Laboratories in a separate Styro and box.
- Put a large note on top of the Styro headed: "LEAKING SPECIMEN." If the specimen was infectious, put all of the contents into a certified container/box with the infectious substance label and write "INFECTIOUS LEAKING SPECIMEN" on top of the Styro.
- On the note, include the courier’s name, whose specimen it was, where the leak or spill occurred, and any other relevant details.
- Be sure to note on the appropriate Specimen Control Document that one specimen from that box has been shipped in a separate Styro and why.
- If a container is leaking inside a bag, immediately secure it inside another Mayo Clinic Laboratories bag and ship it in a separate Styro.
Any leak or spill should be reported immediately to Mayo Clinic Laboratories or the courier’s employer. Do not wait for the end of the run. Call Mayo Clinic Laboratories immediately at 1-800-533-1710 and ask for the Transportation Department. Be sure to communicate whether the courier or anyone else came in contact with the specimen.